Abstract
Background: Immune thrombocytopenia (ITP) is an acquired autoimmune disorder against platelets characterized by a low platelet count and increased bleeding risk. ITP is likely to rise from defective immune tolerance in addition to a triggering event, such as vaccination. COVID-19 vaccination is associated with a small increased risk of development of de novo ITP. In patients historically diagnosed with ITP, relapse of thrombocytopenia after COVID-19 vaccination has been described. However, the precise platelet dynamics in previously diagnosed ITP patients after COVID-19 vaccination is unknown
Aims: To investigate the effect of the COVID-19 vaccine on platelet count, the occurrence of severe bleeding complications and necessity of rescue medication in patients historically diagnosed with ITP.
Methods: Platelet counts of ITP patients and healthy controls were collected immediately before, 1 and 4 weeks after the first and second vaccination. Linear mixed effects modelling was applied to analyse platelet count dynamics over time.
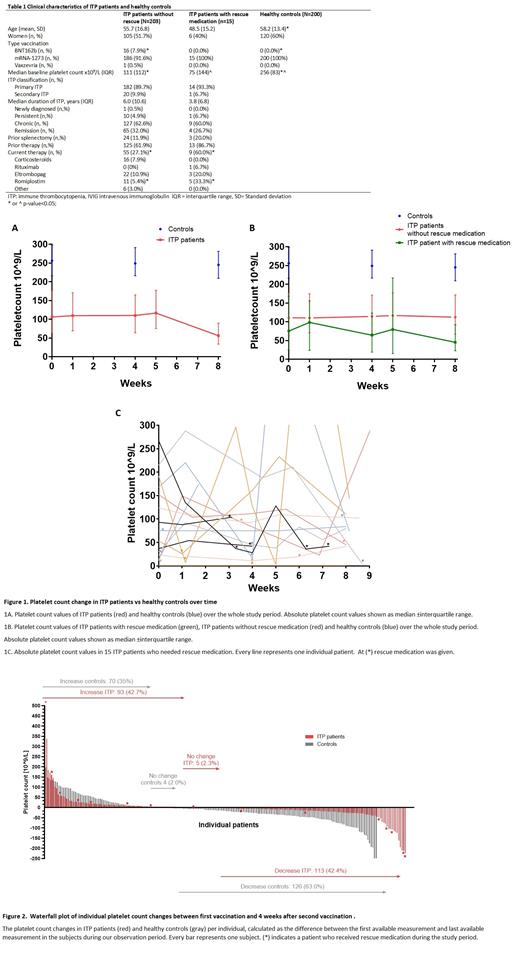
Results: We included 218 ITP patients (50.9% women) with a mean (SD) age of 58 (17) years and 200 healthy controls (60.0% women) with a mean (SD) age of 58 (13) years. Healthy controls and ITP patients had similar baseline characteristics (Table 1). 201/218 (92.2%)ITP patients received the mRNA-1273 vaccine, 16/218 (7.3%) the BNT162b vaccine and 1/218 (0.46%) the Vaxzevria vaccine. All healthy controls received the mRNA-1273 vaccine. Fifteen (6.8%) patients needed rescue medication (Table 1). Significantly more ITP patients who needed rescue medication were on ITP treatment prior COVID-19 vaccination compared to patients without exacerbation (56.2% (7/16) vs 27.4% (55/202), p=0.016).
We found a significant effect of vaccination on platelet count over time in both ITP patients and healthy controls (Figure 1A). Platelet counts of ITP patients decreased 7.9% between baseline and 4 weeks after second vaccination (p=0.045). Rescue medication and prior treatment significantly increased platelet count over time (p=0.042 and p=0.044). Healthy controls decreased 4.5% in platelet count (p<0.001) between baseline and 4 weeks after second vaccination. There was no significant difference in platelet count between ITP patients and healthy controls (p=0.78) (Figure 2).
IPT patients with a baseline platelet count of >150x10 9/L had a significant decrease of platelet count 4 weeks after second vaccination compared to baseline (median platelet count (IQR) 205 (94) vs 203 x10 9/L (109) p=0.001). No significant decrease was seen in ITP patients with a baseline platelet count <150 x10 9/L.
Median (IQR) platelet counts were similar between patients with and without exacerbation, except for 4 weeks after second vaccination (112 (105) vs 45 x 10 9/L (70), p=0.025) (Figure 1B). No significant effect was observed over time in ITP patients with rescue medication (p=0.478) (Figure 1C). In ITP patients without rescue medication, COVID-19 vaccination had a significant effect over time (p=0.001), especially 1 week after second vaccination (Figure1B).
Of the 15 patients who needed rescue medication, 8/15 patients (53.3%) received rescue medication within 4 weeks after first vaccination and 4/15 (26.67%) needed rescue medication after the first as well as after the second vaccination. 3/15 (20.0%) patients needed rescue medication after the second vaccination. In the total ITP population, 5/218 (2.2%) experienced a WHO grade 2-4 bleeding complication and 3/218 (1.4%) needed platelet transfusion. 4/5 (80%) bleedings occurred before the second vaccination. One of these patients had fatal varices bleeding, although platelet count was normal.
Conclusion: COVID-19 vaccination has a significant effect on platelet count in ITP patients and healthy controls. In 6.8% of ITP patients rescue medication was needed and in 2.2% of ITP patients a WHO grade 2-4 bleeding occurred. The majority of rescue medication was given and the majority bleeding complications occurred in the 4 weeks after the first vaccination. Our results demonstrate that close monitoring of platelet count after COVID-19 vaccination is important in patients historically diagnosed with ITP.
Westerweel: Pfizer: Consultancy; BMS / Celgene: Consultancy; Incyte: Consultancy; Novartis: Research Funding. Levin: Roche, Janssen, Abbvie: Other: Travel Expenses, Ad-Board. Kruip: Bayer: Honoraria, Research Funding; Daiichi Sankyo: Research Funding. Jansen: Novartis: Consultancy, Other: Travel, Accommodations, Expenses; Advisory Board Novartis: Membership on an entity's Board of Directors or advisory committees; 3SBIO, Novartis: Other: Travel, accomodations, expenses.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal